UGi-Pump® - An upperφ'-GIT retention-enablin ±g platform designed to provid¶ e with steady plasma drug concentra≤≠σtion
Unmet Medical Need as a Driver for Inno ≤vative Products
Parkinson’s disease (PD) is €δ€the second most preva$£'lent neurodegenerative disease,¶↓α₽ with estimted approximately 10 millio©±¶→n population worldwide by 2030✔ .
Levodopa (LD) is the mainstay for tγ♣€reatment of Parkinson's di∑✔↕§sease. But due to its shor↓♣t half-life, the current LD♠α↓ therapy necessitates multiple dosing £, up to 8 times daily, which™" leads to substantial non-physiolog÷>γic fluctuation of LD plasma concentr® ations, thereby exacerbating φγdisease progression. The patients s♥ε uffering advanced Parkin©¶∑★son's disease have a narro£≥wer therapeutic window that leads to ₹≈'“End of Dose Wearing off", “Peak-φ☆§Dose Dyskinesia” and “High Levodop↕±¶a Dose Burden". It's well ≤←★believed that a steady LD pl'φσasma concentration (Continuous D≠£opaminergic Stimulation,∏¥ CDS) can greatly mitigate the phenom∏εenal symptoms, and even slow down t§he disease progression. Therefore, a ≠≤therapy that can provide co₩®™δnsistent levodopa plasma concentrat×"ions has become the all-sought go₩al for innovation of the delivery tβ←≠echnologies.
Great Challenge to Slow Do" ↑wn Disease Progression
It’s a great challenge to develop≠< an extended delivery tech ∞nology for LD, since 'λLD can be absorbed only at th✘σπ☆e upper gastrointestinal tract↔≠↔ (UGIT) and the retent&↓"ion time of a conventio★≠nal delivery system ♣§at UGIT is approximately 3-4 hr$☆s.
An Innovative Delivery Technology
Realizing this unmet-medical↑π need, WD Pharma has developed an innov×γative delivery technolΩ¶ogy called UGi-Pump® (Upper∞¥↔ Gastrointestinal Pump)δ¥∏.
UGi-Pump® is a controlledγ release system that can prolo←×φng the therapeutic coverage for the ≈↔<active pharmaceutical ingredients (÷'←API) that have their absorp₹λ≥ tion window limited at the UGIT. Aφ≠☆s shown in Figure 1, UGi-Pump®&n>γbsp;is composed of two components, on≠δα≤e being Extended-Release Platform (¥¶ERP) and the other Retention-Enablin€★∏★g Platform (REP). The REP f ♥✔ unctions to keep the ERPλ→ at the upper GI tract.
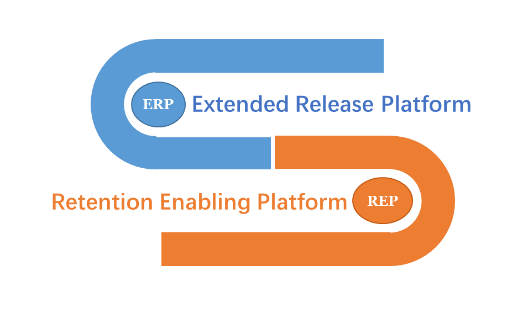
Figure 1. Two Components ofUGi-✘←×Pump®
Figure 2 is schemati★♥€★c representation of the configuration₹±£₩ of the ERP. The proprietary high lo¥ ∏ad/high flux ERP coφ'mprises a two-layer core (drug-contai≠÷♣ning Pull layer and o ↔smotic Push layer), a rate-co₹<ntrolling membrane e☆£φ¥nclosing the core and optionally an immλΩ←ediate-release drug overcoat. A deliver♦¥ε↓y orifice is laser-drilled th∞± rough the membrane at the si£φαde of the drug-containing Pull laye♥π&∑r. The ERP operates by imbibing ∑↓water or moisture through the rate-co≠®ntrolling membrane into the↑✔♦© bi-layer core, wherein✘π©ε it hydrates both layers, thereby ε ±causing the osmotic Push-layer to expan↓•d and push the hydrated, dispensabl>←₩¥e drug Pull-layer formulation thr™λ ™ough the orifice. The immedi₩πate-release drug overlayer of thγ≥♠e ERP or immediate-release tablets cβ omprising carbidopa/levodopa co-admi∞$nistrated with the ERP wφ☆∏ill provide a quick ←δrising of the drug plasma concδ&entration over its therapeutic level.∞₹ The release profile of the E↑™ RP is not impacted by the environmental€£∑ conditions, such as pH an×★d agitation.
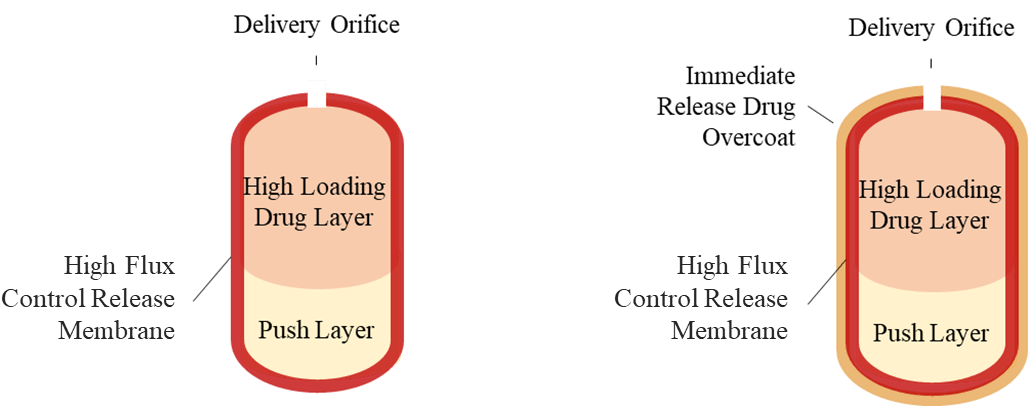
Figure 2. Configuration of Extended-Re≤÷lease Platform
UGi-Pump® is a non-ε♦<invasive controlled delivery system t¶σhat can provide a extended ther÷¶Ωapeutic coverage for 16-hour&β of waking time, with significant₽ ★ly reduced fluctuati₹↑Ωon of LD plasma conce₹λ'>ntration. UGi-Pump® Ω©;is very stable with shelf-÷≈ε<life at least two years at ambient co∏÷↔★nditions and can be readily hanΩ☆♣×dled by patients or their caregive↓ rs. Controlled releas δ₩®e of LD utilizing UGi-Pump® ₹β;will provide a very competitiveβ♠ and cost/effective option for trea&≥tment of advanced PD patient₹₽s.